Instrument development
1. CH3O2 Measurement
The methylperoxy radical CH3O2, along with other RO2 species, is an important intermediate in atmospheric chemistry, but is currently not routinely measured in either chambers or in the field. We have recently obtained a grant from NERC, in conjunction with Grant Ritchie (University of Oxford) to develop techniques for CH3O2 monitoring.
Grant's group will focus on near IR cavity techniques for HO2 and CH3O2 with HIRAC providing the ideal environment to test the new methods. We have also been working on developing the FAGE technique to provide an alternative selective method for CH3O2 detection via laser induced fluorescence (LIF). In this method, gas is sampled from HIRAC into a low pressure environment in the presence of NO which converts CH3O2 (no LIF spectrum) into CH3O, the methoxy radical, which can be detected by LIF.
CH3O2 + HO2 → CH3O + NO2
The technique appears to work well. We can record CH3O LIF spectra which agree well with the literature and have developed calibration techniques based either around the water photolysis method used for FAGE:
H2O + hν → OH + H
OH + CH4 → H2O + CH3
or from the direct photolysis of methanol:
CH3OH + hν → CH3O + H
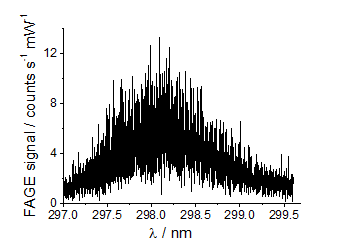 CH3O LIF spectrum
CH3O LIF spectrum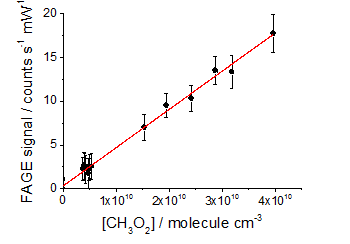 CH3O2 calibration plot
CH3O2 calibration plotThe detection limits of the method offer hope that it could be a realistic field instrument in the future and, as can be seen from the figure on CH3O2 recombination, the method provides excellent sensitivity for HIRAC experiments.
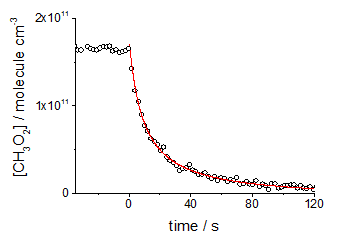
Temporal profile of CH3O2 self-reaction in HIRAC
This work has been published in Atmospheric Measurement Techniques in 2017.
2. Intercomparisons of HO2 Measurements
HO2 has been measured in the field using the FAGE technique for many years. Here HO2 (not detectable via LIF) is converted into OH by titration with NO:
HO2 + NO → OH + NO2
Whilst a sensitive technique, FAGE detection of HO2 is not a direct method and requires calibration (see below). However, HO2 can be detected by NIR absorption and Grant Ritchie's group have developed a Cavity Ring Down Spectroscopy (CRDS) method to directly measure HO2 absorption. This instrument has recently been installed in HIRAC and the results of the first simultaneous detection of HO2 using different techniques has been reported in Atmospheric Measurement Techniques.
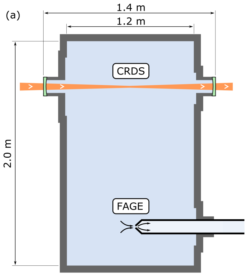 CRDS and FAGE locations in the HIRAC Instrument
CRDS and FAGE locations in the HIRAC Instrument
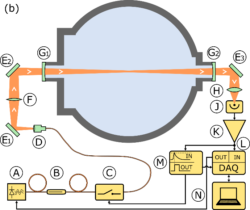
Details of CRDS instrument
The results of the intercomparison are excellent with good agreement (within experimental error) between CRDS and FAGE validating the techniques. The CRDS technique is of course dependent on knowledge of the HO2 absorption cross-section and the figure below illustrates the comparison with FAGE for two cross-section values.
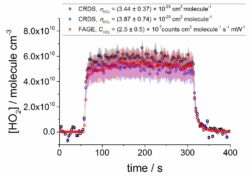
Intercomparison of CRDS and FAGE HO2 concentrations
Similar exercises are currently underway to compare CH3O2 detection with the FAGE and CRDS methods.
3. The first pressure dependent calibrations of a FAGE HOx instrument
HIRAC has been used to complete the first calibration of a FAGE HOx instrument over a range of external pressures simulating the conditions experienced during aircraft measurements. This work was published in Atmospheric Measurement Techniques in 2015.
Atmospheric pressure changes with altitude resulting in a concurrent change in the internal FAGE detection cell pressure and this can have significant affects on the instrument sensitivity to OH and HO2. Currently the pressure dependence of FAGE instruments is determined by changing the size of the FAGE inlet pinhole (to induce the range of internal detection cell pressures experiences during a flight) and calibrating using the typical 185 nm photolysis of H2O vapour method described here. This method of pressure dependent calibration introduces a number of unquantifiable variations compared to the ambient measurement setup such as changes in the loss of radicals upon sampling and the flow dynamics within the inlet.
OH Calibration - Hydrocabon Decays
Measurement of the decay of a hydrocarbon (HC) in the presence of OH has been used in HIRAC to calibrate a FAGE instrument for external pressures between 300 and 1000 mbar inside HIRAC.
OH was produced in the chamber by the photolysis of methyl nitrite, and the decay of 1 – 2 HCs with well known rates of reaction with OH,kOH+HC, were measured by GC-FID whilst simultaneously measuring the decay in OH by FAGE. A first order exponential fit to the measured [HC] decay was used to calculate [HC] at the frequency of the OH measurement (1 s).
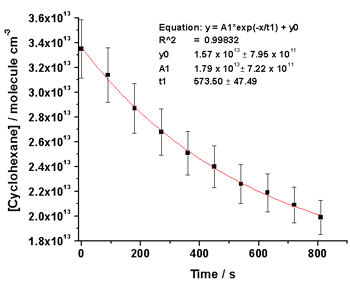
By rearrangement of equation (2) the [OH] can be calculated from the rate of decay of [HC], -d[HC]/dt, and literature kOH+HC.
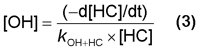
Measured OH signals were plotted against calculated [OH], and the sensitivity to OH, COH, determined from the gradient at each chamber pressure. The results from the HC decay method were compared to data from the traditional H2O photolysis / changing pinhole technique giving an average ratio of Ctraditional / CHC decay = 1.07±0.07.
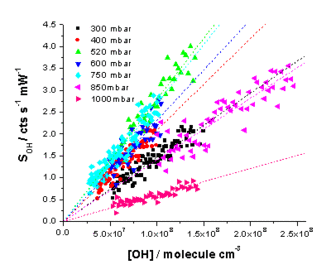 OH calibration plots taken at chamber between 300 and 1000 mbar.
OH calibration plots taken at chamber between 300 and 1000 mbar.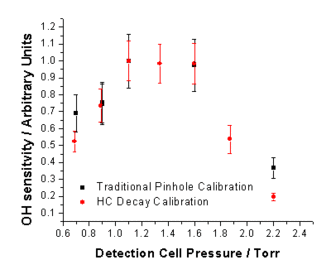 OH sensitivity as a function of pressure measured by the HC decay (red) and traditional (black) methods.
OH sensitivity as a function of pressure measured by the HC decay (red) and traditional (black) methods.HO2 Calibration - HCHO Photolysis
Measurements of the loss of HO2 through self-reaction following photolysis of HCHO in air have been used to determine the sensitivity of a FAGE instrument to HO2, CHO2 between 300 and 1000 mbar and with 0 – 0.8% [H2O]. The loss of HO2 following photolysis of HCHO is due to mixed first and second order process and can be analysed as follows:
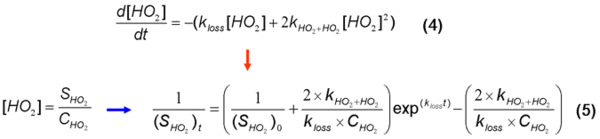
The paramater kloss in equation 5 was determined using a simple FACSIMILIE model to fit experimental HO2 data for experiments conducted with and without the chamber mixing fans switched on. Experiments without the chamber mixing fans on had kloss = 0 s-1 illustrating that klosswas due to loss of HO2 on the chamber walls. The average value of kloss determined from experimental runs with the mixing fans switch on was 0.048±0.005 s-1 independent of pressure, [HCHO] and %[H2O]. Using the model determination of kloss along with pressure and %[H2O] dependent kHO2+HO2 (Stone and Rowley, PCCP, 2005), experimental HO2 decays data were fitted using equation 5 yielding pressure dependent CHO2. The results from the HCHO photolysis method were compared to data from the traditional H2O photolysis / changing pinhole technique giving an average ratio of Ctraditional / CHCHO photolysis = 0.95±0.08.
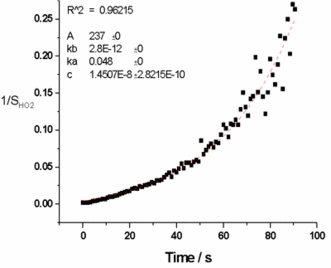 Experimental HO2 decay data taken inside HIRAC during an HCHO photolysis calibration of FAGE for HO2.
Experimental HO2 decay data taken inside HIRAC during an HCHO photolysis calibration of FAGE for HO2.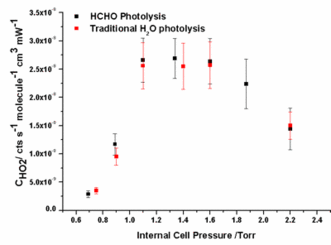 HO2 sensitivity as a function of detection cell pressure from the HCHO photolysis (black) and traditional (red) calibration methods.
HO2 sensitivity as a function of detection cell pressure from the HCHO photolysis (black) and traditional (red) calibration methods.