Mechanism development
1. OH yields from O3 + alkene reactions
The OH radical is the major oxidant of volatile organic compounds (VOCs) in the atmosphere. Ozone alkene reactions are a non-photolytic source of HOx radicals and appear to be a significant source of new HOx radicals in urban and rural (especially forested) air. The dark reactions between O3 and trans-2-butene and isoprene have been studied in HIRAC. Only results from the O3+isoprene study will be discussed here but further information about the O3+trans-2-butene results have been published by Glowacki et al. (2007).
Pressure dependent (100 – 1000 mbar) OH yields from O3+isoprene have been measured both directly and indirectly in HIRAC.
Indirect measurements
Scavenger technique: Using cyclohexane as an OH scavenger, the yield of cyclohexanone was measured using GC-FID along with the concurrent loss rate of isoprene.
Kinetic technique: Kinetics studies using cyclohexane. A 10 fold excess O3 over isoprene was used to ensure that the decay of isoprene was pseudo-first order. The consumption of isoprene was monitored using both FTIR and GC-FID.
Direct measurements
OH and HO2 yields were measured using FAGE for chamber pressures between 300 and 1000 mbar. A simple kinetic model based on a subset of the Master Chemical Mechanism (MCM) was used to model the OH and HO2 concentrations and hence determine pressure dependent OH and HO2 yields.
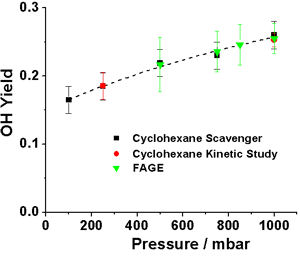 Pressure dependent OH yields determined by indirect and direct techniques.
Pressure dependent OH yields determined by indirect and direct techniques.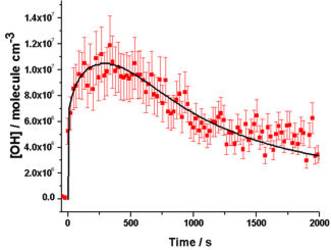 Direct FAGE OH measurements p = 1000 mbar (red) with modelled data (black) giving YOH = 0.26±0.02.
Direct FAGE OH measurements p = 1000 mbar (red) with modelled data (black) giving YOH = 0.26±0.02.2. Product formation yields from O3 + isoprene reactions
Recently, more investigations were performed studying the temperature dependence of the OH and HO2 yields from the isoprene + O3 reactions over the range of temperature 0 – 70 °C. The work is in progress and preliminary results were presented at International Symposium on Gas Kinetic, Szeged, 2014 (Bejan et al., 2014, poster).
Product formation yields of methacrolein and methylvinylketone as the main stable products formed in the ozonolysis of isoprene have been recently investigated using the facilities offered by HIRAC chamber (wide range of temperature, FTIR, GC).
Preliminary results are presented below for one of the products investigated.
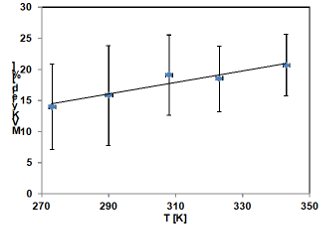
methylvinylketone yield from the ozonolysis of
isoprene using cyclohexane as OH scavenger at
1000 mbar of air and 5 different temperatures.
3. OH Production in the Reaction of CH3C(O)O2 + HO2
One of the ‘hot topics’ in atmospheric chemistry is the observation of much higher than expected levels of the OH radical in tropical forested environments. As the Tropics are the region where most of the greenhouse gas methane is removed via reaction with OH, it is vital to have accurate measurements of OH concentrations in these regions. Interferences with the measurements could explain some of the observations and using HIRAC to calibrate and characterise FAGE (OH and HO2 measurements) is important part of the work we do.
Unknown chemistry could also be contributing to OH production and one postulated mechanism is the reaction of certain RO2 with HO2 which had previously been considered as a radical sink. Indirect studies in 2004 by Hassoon et al. suggested a substantial OH yield from the title reaction, but this remained controversial. However, more recently Dillon and Crowley positively identified OH. This study is however, the first time in which products from all three channels of the reaction have been positively identified.
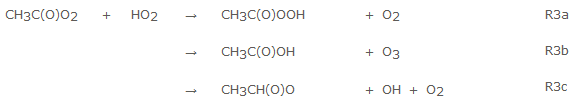
HO2 and OH are followed using FAGE, peracetic and acetic acid via FTIR and O3 via the calibrated O3 box. Below are some product profiles. Our best estimates of the yields are: αR3a = 0.35 ± 0.03, αR3b = 0.12 ± 0.02 and αR3c = 0.54 ± 0.09. This work has been published in Atmospheric Chemistry and Physics in 2016. For more information see the poster section.
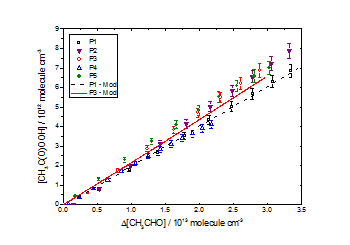 CH3C(O)OOH as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ
CH3C(O)OOH as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ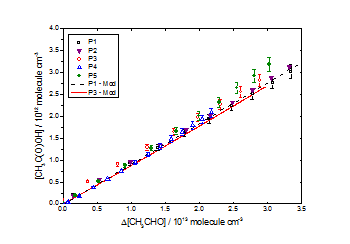 CH3C(O)OH as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ
CH3C(O)OH as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ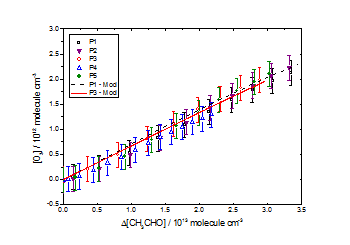 O3 as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ
O3 as a function of Δ[CH3CHO] for [CH3OH]0:[CH3CHO]0 ≈ 3.8 in air at 1000 mbar and 293 K. Good agreement was observed between experimental data and the chemical model for all datasets with the above yields. All uncertainties quoted to ± 1σ4. HO2 + Carbonyls and Subsequent Reactions
An important secondary process in the CH3C(O)OO + HO2 is the reaction of HO2 with formaldehyde. This process is reversible with complex formation increasing with lower temperatures:
HO2 + HCHO ↔ HOCH2O2
Reaction of this peroxy radical with HO2 can also lead to OH production.
HO2 + HCHO ↔ HOCH2O2
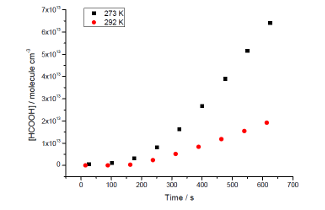
Preliminary studies have observed direct OH production and the figure below shows the dramatic increase in secondary HCOOH (formic acid) production as the temperature is lowered. The temperature control available to HIRAC is critical to these studies.