Kinetic Studies
1. Time Resolved Studies of CH3O2 Recombination and Reaction with HO2
Using our new FAGE based methods for CH3O2 detection we are currently looking at the kinetics of methylperoxy recombination reactions and the reaction with HO2. Concurrent FTIR measurements of formaldehyde and methanol are also being used to assess the branching ratio of the recombination reaction.
CH3O2 + CH3O2 → CH3O + CH3O + O2 or CH2O + CH3OH + O2
The methoxy radical produced reacts with oxygen to generate HO2 and H2CO, so it is actually not possible to study the recombination reaction in the absence of some contribution from HO2 chemistry. However, the fact that we can directly measure HO2 by FAGE is a significant advantage of our methodology.
Previous studies have primarily focused on UV spectroscopic detection of RO2 and HO2, this method is both less sensitive and due to the broad, featureless spectra, less selective.
The figure below shows an example of the quality of decay traces that can be obtained with the FAGE based apparatus and we hope to be presenting and publishing material on these reactions shortly.
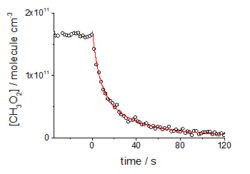
CH3O2 decay due to self reaction and cross-reaction with HO2
2. Kinetics and Structural Activity Relationship (SAR) development
Relative rate studies of the reaction of Cl atoms with a series of acetates
Accurate kinetic data for the reactions of chlorine atoms with esters are needed for the analysis of data from smog chamber experiments in which chlorine atoms are used to initiate the oxidation of organic compounds. Relative rate studies of the reaction of Cl atoms with a series of acetates have been carried out inside of HIRAC using GC-FID and FTIR spectroscopy and the results of these experiments are tabulated below. The table also shows comparisons between the experimental values and the those calculated using the Structural Activity Relationship (SAR) method.
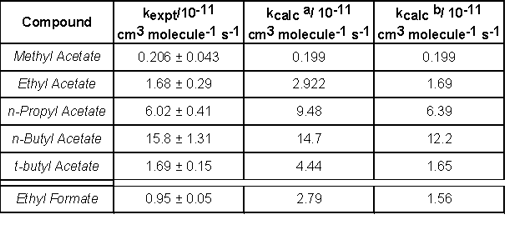
Cl atoms with the studied acetates along with values calculated using the SAR
method. Calculated using factors aF(-CO) and bF(-C(O)O-).
3. Relative Rate Study on the Reactions of Cl and OH with Cyclohexanes
For the alternative OH calibration methods we want to use hydrocarbons with as large rate coefficients as possible, whilst still retaining ‘simple’ chemistry. Reactions with alkanes are ideal candidates as the reaction takes place via a direct abstraction and the alkanes are not susceptible to photolysis and have low surface losses. Cycloalkanes are especially good with a significant number of secondary C-H bonds and cyclohexane has been used in some of our studies. The presence of even weaker tertiary C-H bonds in alkyl substituted cycloalkanes (e.g. methylcyclohexane) may increase rate coefficients even more. We have therefore undertaken a study as part of Jamie Kelly’s M.Chem project, to look at the rates of Cl and OH with a series of alkylcyclohexanes.
The figure below (Fig. 2a) shows a typical relative rate plot for methylcyclohexane with cyclohexane as the reference compound and with two different OH sources (ozonolysis of alkenes and CH3ONO photolysis). The experiments have been repeated at a number of temperatures and the resulting Arrhenius plot is shown below (Fig. 2b).
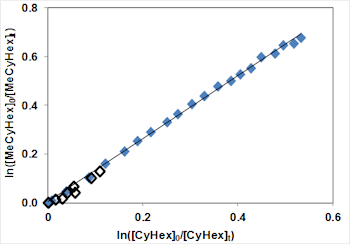 Fig. 2a: Relative rate plot for methylcyclohexane with cyclohexane as the reference compound.
Fig. 2a: Relative rate plot for methylcyclohexane with cyclohexane as the reference compound.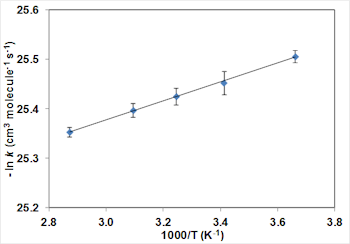 Fig. 2b: Arrhenius plot for relative rate experiments of methylcyclohexane with cyclohexane as the reference compound.
Fig. 2b: Arrhenius plot for relative rate experiments of methylcyclohexane with cyclohexane as the reference compound.The results suggest that alkylation of the cycloalkane makes relatively little difference to the rate coefficients, presumably due to the positive effects of tertiary C-H bonds being off-set by increased steric hindrance.
4. Relative Rate Study on Cl atom Reactions with Methylketones
Since the observation of enhanced concentrations of Cl atom precursors over continental environments, there has been increased interest in the role of Cl atom chemistry in VOC removal. Daytime Cl atom concentrations will always be lower than OH, but the enhanced reactivity of Cl over OH, means that Cl atoms can still contribute to VOC removal. In addition, Cl atom chemistry is often used to initiate reactions in chambers.
In this study we have looked at the reactions of a range of methylketones with Cl atoms at room temperature and atmospheric pressure using the relative rate technique. The figure below shows an example of a typical relative rate plot. The data reported are the average of several experiments and we try to use at least two reference compounds to avoid interferences from possible systematic errors (e.g. unnoticed overlapping peaks in the FTIR spectra).
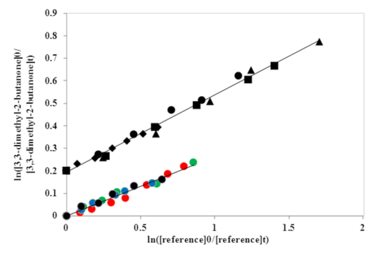
Comparison with previous data (where available) indicated some possible errors in earlier flash photolysis studies which may have been influenced by chain chemistry. Additionally, agreement with predictions from previous Structure Activity Relationships (SAR) was also poor for some compounds (particularly 3-methyl-2-pentanone and 3,3-dimethyl-2-butanone) and therefore we created a modified SAR activity where the agreement between experiment and prediction is much closert to that shown schematically in our TOC graphic!
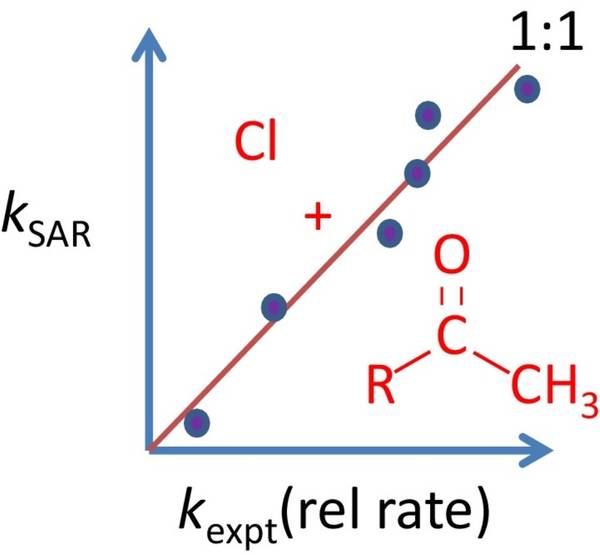
Full details can be found in Farrugia et al. Chemical Physical Letters 640 (2015) 87-93.
