FAGE
Fluoresence Assay by Gas Expansion, FAGE, is a low pressure, on-resonance, laser induced fluoresecence technique used for the detection of OH and HO2 radicals. The HIRAC FAGE instrument is based on the design of the University of Leeds aircraft FAGE instrument installed on the BA146 FAAM research aircraft.
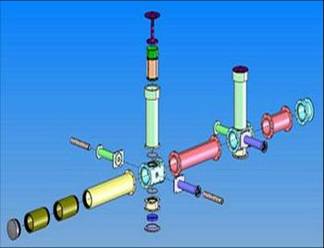 Solids Works diagram of the HIRAC FAGE design.
Solids Works diagram of the HIRAC FAGE design. Picture of the HIRAC FAGE setup and the calibration wand.
Picture of the HIRAC FAGE setup and the calibration wand.Detailed information about the FAGE technique and instrument calibrations can be found in Prof. Dwayne Heard's and Prof. Mike Pilling's review of OH field measurements by LIF. There is also lots of interesting information and further references about the University of Leeds ground-based and aircraft FAGE instruments on the FAGE group website. For a brief description of the FAGE technique and HOx calibrations see below.
How does FAGE work?
- Air from the HIRAC chamber is drawn down the FAGE sampling inlet into the low pressure (~1 Torr) detection cells through a small pinhole nozzle (<1 mm). The length of the sampling inlet can be adapted allowing HOx measurements at a number of points across the radius of the chamber.
- After 30 - 60 cm the air sample reaches the first detection axis where OH radicals are detected by LIF. OH radicals are excited by the fibre coupled, second harmonic Ultra-Violet output from a Nd:YAG pumped dye laser at ~308 nm.
- The resultant OH fluorescence, also at 308 nm, is collected by a gated channeltron photomultiplier on the axis perpendicular to the laser beam and is then analysed by gated photon counting.
- HO2 radicals are detected indirectly in the second detection axis following chemical conversion to OH using a small flow of NO (HO2 + NO → OH + NO2).
In HIRAC OH and HO2 data are collected with a time resolution of ~1 s at which the instrument has detection limits of the order 106 to 107 molecule cm-3 for OH and HO2.
Calibrating FAGE
The FAGE technique is not absolute, requiring calibration to measure the instrument sensitivity to OH (or HO2), COH (or CHO2), which is then used to convert the measured OH signal into a concentration:
![]()
Calibrations are carried out using the using the photolysis of water vapour by the 185 nm output from a mercury pen-ray lamp which gives OH and HO2 in a 1:1 ratio.
Humidified, ultra-high purity air is flowed at 40 slm (standard litres a minute) down a small rectangular flow tube shown in the figure below and is photolysed through a Suprasil window by the 185 nm output from a mercury lamp.
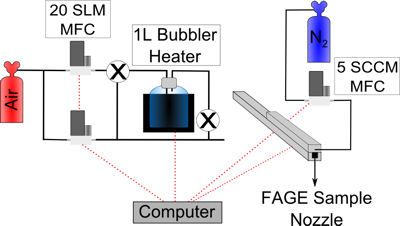
Schematic of the FAGE calibration setup.
The output from the flow tube is then sampled by the FAGE instrument. The concentrations of OH and HO2 produced during calirbrations can be calculated using the following equation:
![]()
where [H2O] is the measured concentration of water in the humidified air, σH2O, 185nm is the well-defined cross-section of water at 185 nm, φOH is the photodissiociation quantum yield of OH radicals at 185 nm, F185nm is the flux of 185 nm light from the mercury lamp which is measured using N2O actinometry and t is the irradiation time. By plotting the measured OH (or HO2) signal as a function of the concentrations produced during a calibration a straight line plot with a gradient equal to COH (or CHO2) is gained.
The development of an alternative calibration systems for OH (based on reactions with hydrocarbons) and for HO2 (based on self-reaction) have formed part of recent undergraduate and PhD projects. These new calibration systems have allowed us to test the pressure dependence of the FAGE instrument sensitivity.
