OH Reactivity
Measuring the total loss of OH in a sample provides further important constraints for modelling. Measuring an absolute concentration of OH is of course vital, but the model can potentially reproduce this concentration with an infinite combination of different production and loss rates. OH reactivity measurements provide an experimental constraint on the loss term, and hence in conjunction with a measured absolute [OH], provide a rate of production term.
In the Leeds reactivity chamber schematically shown below, OH is generated via pulsed photolysis of ozone, with the O(1D) generated rapidly producing OH radicals. The decay of the photolytically generated OH is then followed in real time by coupling the reactivity chamber to a FAGE cell.
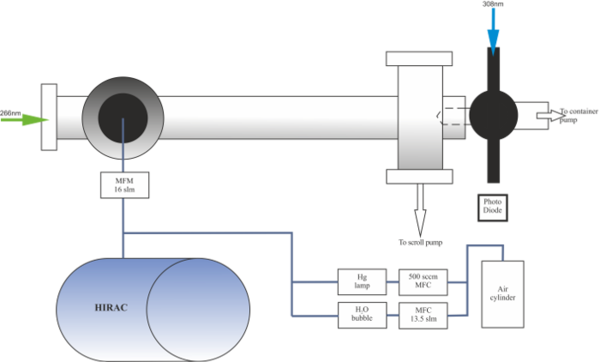
Schematic of the OH reactivity instrument set up for sampling from HIRAC.
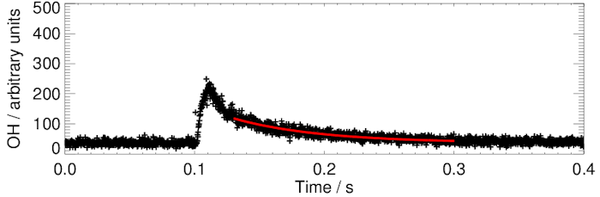
Typical OH decay
The measured first order loss of OH, k’, is the combination of the loss processes with all the different species, i, in the mixture, e.g. for CO this is kOH+CO[CO]. The total loss is given by:
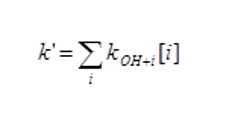
If the concentration of all the species are known (e.g. via GC measurements) then the OH reactivity can be calculated and compared with the experimental value. Any underestimation of the loss would suggest the air sample contains species that are not being measured (or measured correctly).
As part of her PhD project, Charlotte Brumby is interfacing HIRAC to the Heard Group’s Reactivity Instrument. Preliminary results are successful and have been presented at the International Gas Kinetics Symposium in Szeged in July 2014. The controlled conditions of HIRAC provide an ideal environment to test and validate the Reactivity Instrument.
Charlotte is interested in studying the atmospheric oxidation of biofuels (e.g. ethanol) and biofuel combustion emissions in HIRAC and in using the reactivity instrument to investigate changes as the material is processed. For example as the relatively unreactive ethanol is oxidized to more reactive ethanal, the measured reactivity will significantly increase.
